Annual Benefits Report 2023 | Veterans Tinnitus

The US Department of Veterans Affairs (VA) released the 2023 Annual Benefits Report (ABR). ABR offers insights and data on Veterans Benefits Administration (VBA). Veterans Tinnitus is the #1 service-connected disability in the 2023 VBA.
- Did you know? Tinnitus has been the #1 service-connected disability since the Vietnam War (1955 – 1975).
This post will highlight key takeaways from the 2023 VA ABR. As well as this, this post will explore tinnitus causes and treatments and much more.
- What is Veterans Affairs?
- What is the Annual Benefits Report?
- ABR Takeaways
- What is Tinnitus?
- What Causes Tinnitus?
- Is there a Cure for Tinnitus?
- What is Lenire?
- Is Lenire Effective?
What is the US Department of Veterans Affairs?

US Veterans Affairs provides lifelong healthcare services to Veterans. As well as this, the VA provides benefits and cemetery services.
The VA operates 170 VA medical centers and outpatient clinics. There are 1,321 health care facilities. These facilities service 9 million enrolled US Veterans every year. At a high level, ABR outlines how Veterans interact with VA services.
What is the Annual Benefits Report?

The Annual Benefits Report summarizes VBA programmes. In addition, ABR identifies VBA engagement, and profiles those who benefit.
The ABR aims to achieve the following:
- Show a clear data-driven picture of how Veterans and their dependents use benefits.
- Provide insights into the nature of the VBA programmes.
You can read the US VA Annual Benefits Report on the US VA’s website. Keep reading for some takeaways from the US VA’s Annual Benefits Report.
ABR Takeaways
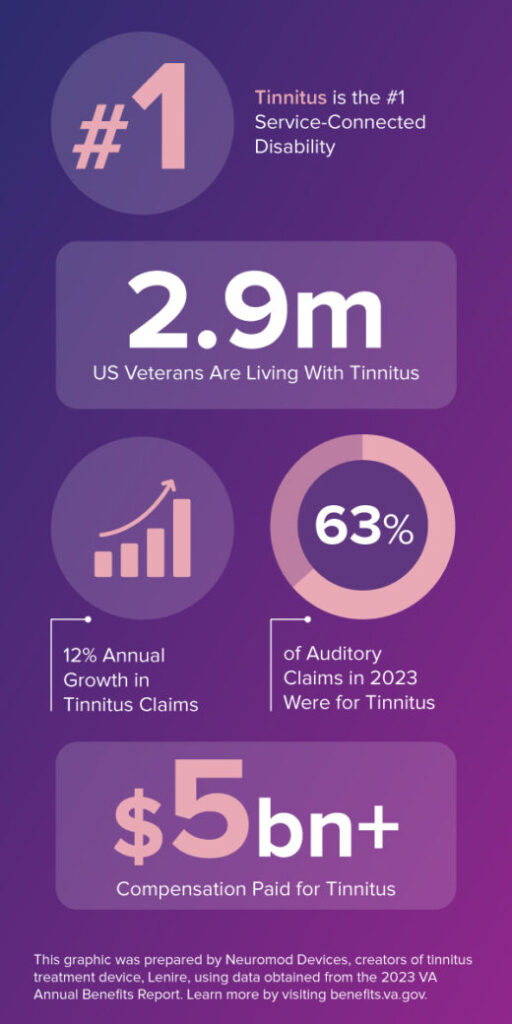
ABR covers the federal benefits for Veterans and dependents. The infographic above shows how Veteran tinnitus prevalence.
- #1 Service-Connected Disability is Tinnitus
- 2.9m US Veterans Are Living With Tinnitus
- 12% Annual Growth in Tinnitus Claims
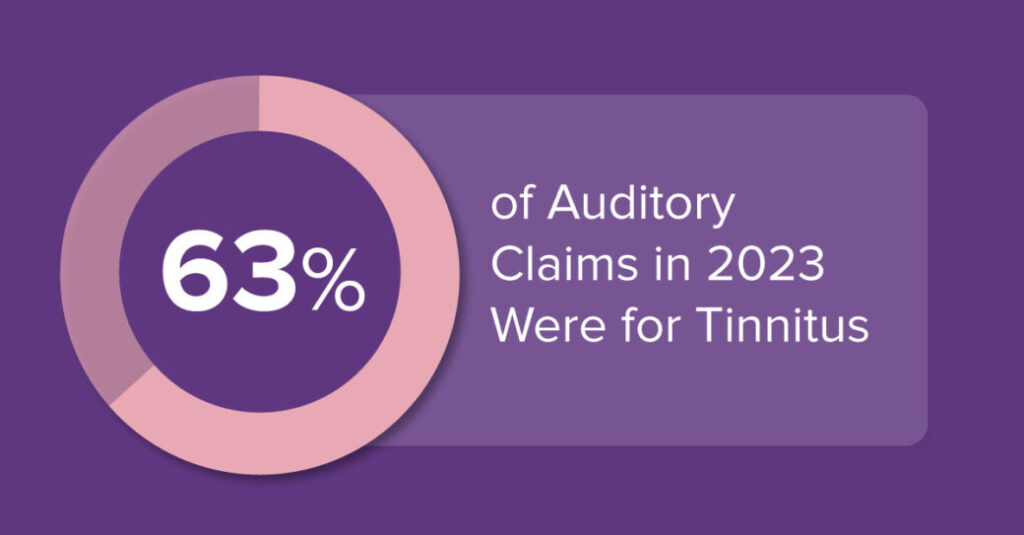
- 63% of Auditory Claims in 2023 Were for Tinnitus
- $5.8bn Compensation Paid for Tinnitus
As referenced, tinnitus has been the #1 service-connected claim since the mid-1950s. The VA processed 2.7m claims for tinnitus in 2022. This number grew to 2.9m Veterans with tinnitus in 2023. ABR 2023 indicates double-digit growth each year.
What is Tinnitus?

Tinnitus is when a person hears noise with no outside source. It is often described as high pitched ringing in the ears. Tinnitus can sound like hissing, buzzing, and many more types of sound. An estimated 15% of the global adult population lives with tinnitus.
Noise exposure is likely most people’s first brush with tinnitus. Noisy events such as concerts can result in short-term tinnitus. In the majority of these cases, tinnitus fades over time. On the other hand, many people have long-term tinnitus.
Objective and subjective tinnitus are the two types of tinnitus.
- Subjective Tinnitus: 99% of all tinnitus cases are subjective. Only a person with subjective tinnitus can hear the noise. As a result, there is no objective measurement of this noise.
- Objective Tinnitus: Objective tinnitus is tinnitus that a doctor can hear when they examine a patient. Blood vessel problems or ear muscle contractions can cause objective tinnitus.
Pulsatile Tinnitus is another rare form of tinnitus that mixes high and low frequency sounds. Pulsatile tinnitus often pulses in time with a patient’s heartbeat. This form of tinnitus can be objective or subjective.
What Causes Tinnitus?
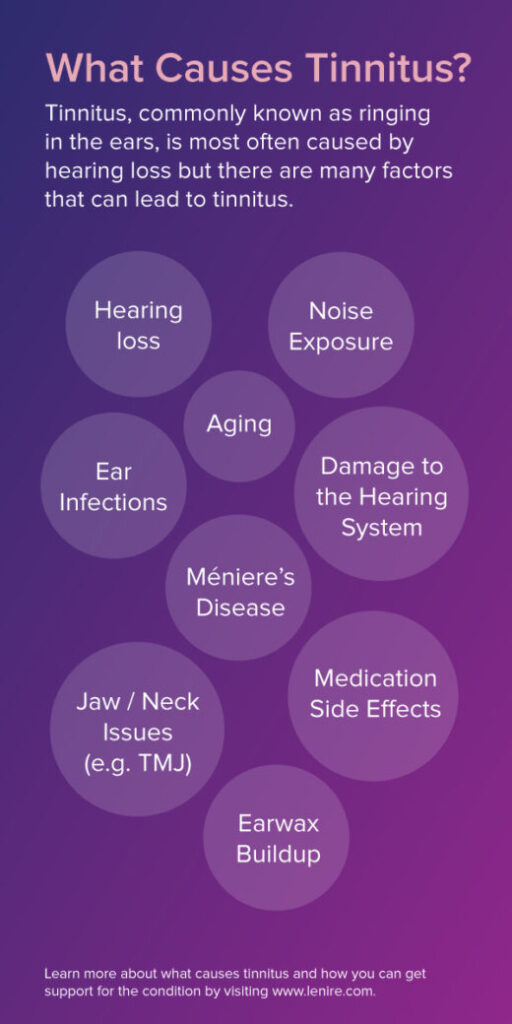
Hearing loss is the number one cause of long-term tinnitus. There are many additional causes. These include, but are not limited to the following:
- Hearing Loss
- Ageing
- Exposure to Loud Noise
- Ear Infections
- Méniere’s Disease
- Damage to the Hearing System
- Earwax Buildup
- Jaw / Neck Issues (e.g. TMJ)
- Medication Side Effects
Tinnitus in veterans may have additional factors. Learn more about veterans and tinnitus at the Veterans Health Library.
Hearing loss is the number one cause of tinnitus. Despite this, tinnitus military disability is the #1 claim per the 2023 ABR. 2.9 million US Veterans claimed Tinnitus VA Disability per the 2023 ABR.
VA and DoD created Progressive Tinnitus Management (PTM). You can learn more about PTM on the VA’s website. PTM helps Veterans manage tinnitus but is there a cure for tinnitus?
Is There a Cure for Tinnitus?
No. There is no cure for tinnitus at the moment. There are a number of tinnitus therapies that can provide tinnitus relief. These include:
- Hearing Aids: People with hearing aids may get relief from improved hearing. Many hearing aids also feature a noise masking feature. Noise masking distracts the brain from tinnitus sounds but only while the hearing aid is on.
- Cognitive Behavior Therapy (CBT): CBT helps those living with tinnitus developing coping mechanics for tinnitus. CBT can also help someone manage the stress and anxiety linked to the condition.
- Tinnitus Retraining Therapy (TRT): TRT is a customized therapy plan for tinnitus patients. TRT helps those living with tinnitus develop response and management techniques. TRT can often incorporate sound-masking.
- Bimodal Neuromodulation: A new form of tinnitus treatment that is also known as dual mode. Dual mode stimulates two points of the nervous system at once. Lenire is the only FDA Approved dual mode tinnitus treatment device.
- Earwax Removal: A buildup of earwax in the ear canal can cause or inflame tinnitus. Removing earwax can provide relief.
- Supplements: Clinical data that supports supplements as a tinnitus treatment is limited. You can learn more about supplements by watching Dr. Cliff’s insightful video.
There are a range of additional tinnitus therapies. These include laser treatments, medication, meditation and more. Patients should consult with a hearing care clinic prior to trying any therapy.
You can find a tinnitus expert using Lenire’s Find a Clinic Map.
What is Lenire?

Lenire is a dual mode tinnitus treatment device. The device combines audio and tongue stimulation.
Lenire’s audio plays through wireless headphones. As audio plays, the Tonguetip sends mild pulses to the tongue. This dual mode method retrains the brain to ignore tinnitus.
Lenire is safe for at-home use. Lenire is available through specialized hearing care providers. Providers create custom plans, and guide tinnitus patients through treatment.
Three large scale clinical trials examined Lenire’s effectiveness. These trials involved more than 600 patients.
Is Lenire Effective?

Lenire became the first device of its kind awarded US FDA Approval. The US FDA Awarded Lenire a De Novo Grant. Lenire’s third large scale clinical trial was pivotal for approval. Lenire’s third large-scale clinical trial was TENT-A3.3
During the TENT-A3 Clinical Trial:
- 79.4% of patients had a significant reduction in tinnitus severity.3,4
- 88.6% would recommend Lenire®.3
TENT-A3 showed Lenire to be more effective than sound-only therapy for 70.5%. Patients included had moderate or worse tinnitus.
Lenire’s first large-scale clinical trial was TENT-A1. TENT-A1 is one of the largest clinical trials for tinnitus in history. This trial was one of the longest followed-up tinnitus clinical trials.
TENT-A1 was the cover story for Science Translational Medicine. Science is a top tier peer-reviewed journal. TENT-A1 enrolled 326 participants and found:
- 86.2% of patients reported relief from tinnitus after 12 weeks of Lenire.1,4
- 80.1% of these patients had relief that lasted for at least a year after treatment.1
Lenire’s second clinical trial was TENT-A22. Nature – Scientific Reports published TENT-2’s remarkable results. TENT-A2 showed that changing stimulation midway led to stronger results. TENT-A2 had 191 participants and found:
- 95% of patients reported relief from tinnitus after 12-weeks of Lenire.2,4
- 91% of these reported relief that lasted for at least a year after treatment.2
Researchers asked patients in all trials if they would recommend Lenire. 83% of 500+ patients would recommend Lenire to treat tinnitus.1,2,3 As well as this, real world Lenire Patient Stories received major media coverage.
Learn More About Tinnitus and Veterans Tinnitus
Veterans should speak to their VA Audiologist for a tinnitus and hearing exam. For more on tinnitus, tinnitus research, and dual mode stimulation, sign up for the Lenire Newsletter. Fill out the form below to sign up.
References
- TENT-A1 Clinical Trial
- TENT-A2 Clinical Trial
- TENT-A3 Clinical Trial will be published in 2024.
- Measured using THI.



